Plethodontinae
Allan Larson, David Wake, and Tom Devitt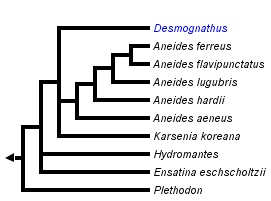

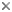
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The subfamily Plethodontinae (sensu Chippindale et al., 2004 and Min et al., 2005) contains salamanders of the genera Aneides and Plethodon, which occur in both eastern and western North America, the western North American genus Ensatina, the Korean Karsenia koreana, the supergenus Desmognathus of the eastern U.S., and the genus Hydromantes of California, southern Europe, and Sardinia (sensu Wake et al., 2005). Aneides, Plethodon and Ensatina inhabit underground retreats where eggs are laid during drier periods, becoming active just beneath the surface under logs and rocks on the forest floor during wetter periods. These salamanders are completely terrestrial and have no free-living aquatic larval stage. Surface activity is highly seasonal for most species and usually occurs on wet nights.
Members of the supergenus Desmognathus are widely distributed in the forests of eastern North America. Three species (Desmognathus aeneus, Desmognathus wrighti and Phaeognathus hubrichti) are strictly terrestrial and lack an aquatic larval stage. Eggs are laid on land and hatch fully formed. All remaining species belong to the genus Desmognathus and have biphasic life histories featuring gilled, aquatic larvae and fully metamorphosed adults. Hydromantes salamanders are associated with caves and rocky areas of relief in California (subgenus Hydromantes) and southern Europe (southwestern France and Peninsular Italy and Sardinia; subgenera Atylodes and Speleomantes).
Ensatina and Plethodon (except Plethodon petraeus) occur primarily on the forest floor and have only limited ability for climbing in low vegetation. Some Aneides species show greater climbing ability and associated arboreality (most highly developed in A. ferreus, A. vagrans, and A. lugubris; Larson et al., 1981) or use of rock crevices (A. aeneus). Morphological changes associated with evolution of climbing in Aneides include lengthening of the limbs and digits, rearrangements of the cartilaginous carpal and tarsal elements, and expansion of terminal phalanges to enhance grasping (Wake, 1963; Larson et al., 1981). Plethodon petraeus (Wynn et al., 1988) demonstrates adaptations for climbing in rock crevices, some of which parallel those of Aneides.
Aneides also demonstrates morphological evolutionary changes that strengthen the jaws, including fusion of premaxillary bones, enlarged unicuspid teeth, dorsoventral expansion of the posterior portion of the maxilla, and rearranged throat musculature (Wake, 1963; Larson et al., 1981: Wake and Larson, 1987). The prominent jaw muscles of Aneides give their head a more massive and triangular appearance relative to the other plethodonine salamanders. These features are often especially pronounced in males. The strengthened jaws and enlarged, sharp teeth of Aneides are used in aggressive encounters among conspecifics (Staub, 1993). When handled, Aneides lugubris occasionally delivers bites that can draw blood but are not seriously dangerous. The strengthened jaws of Aneides have been hypothesized to be adaptations for acquiring food while maintaining a perch in arboreal habitats (Larson et al., 1981).
The genus Hydromantes comprises three clades, one in California (subgenus Hydromantes), and two in Southern Europe and Sardinia (subgenera Atylodes and Speleomantes; Wake et al., 2005). Members of the genus Hydromantes inhabit wet rocky areas and caves, and possess unique adaptations for locomotion on slippery rock surfaces, including flattened digits that are webbed in some species. The short tail is used to assist in maintaining a purchase on the substrate by pushing the tip of the tail against the substrate each time a hind foot is moved forward. One species, Hydromantes platycephalus, exhibits a unique escape behavior. Like many other plethodontids, this species coils tightly into a ball when disturbed or picked up. Unlike any other species however, H. platycephalus will actually roll downhill if dropped (Garcia-Paris and Deban, 1995).
The salamanders of the genus Ensatina have been featured prominently as an example of a ring species (Stebbins, 1949; Dobzhansky, 1958; Futuyma, 1998; Dawkins, 2004; Wake, 2006). Populations of Ensatina eschscholtzii demonstrate extensive variation in coloration, forming seven recognized subspecies arranged geographically in a "ring" encircling the central valley of California. Hybridization and intergradation occur between adjacent subspecies in the ring except where coastal and inland populations make contact at the southern end of the geographic range of the species. This geographic pattern is interpreted to illustrate gradual evolution of reproductive barriers between populations, an important component of the formation of species. Because molecular evolutionary studies illustrate extensive genetic differentiation among populations of Ensatina, some systematists prefer to regard this genus as containing two or more species rather than a single "ring species" (Frost and Hillis, 1990; Highton, 1990).
Geographic variation in reproductive isolation among populations also exists for members of the Plethodon glutinosus and P. cinereus groups (Highton, 1972; Highton et al., 1989, respectively). Plethodon shermani, a member of the P. jordani group, hybridizes with members of the P. glutinosus group (Weisrock et al., 2005). A detailed behavioral analysis of the evolution of sexual isolation within the Plethodon glutinosus group was presented by Reagan (1992).
Members of the subfamily Plethodontinae show unusually high variation in the mass of DNA in the cell nucleus. The C-value (mass of DNA in a haploid, unreplicated set of chromosomes) has been reported to range from 18 pg to 69 pg in the subfamily Plethodontinae with the full range being evident within the genus Plethodon (reviewed by Larson, 1984; Sessions and Larson, 1987; Gregory, 2006). Mass of the nuclear genome may be inversely proportional to developmental rates, especially rates of differentiation of regenerating limbs (Sessions and Larson, 1987).
Characteristics
Detailed Characteristics of the Subfamily Plethodontinae
Characteristics are summarized from Lombard and Wake's (1986) phylogenetic analysis of major plethodontid lineages based on 30 morphological characters with special emphasis on the hyobranchial skeleton and musculature of the feeding system. These characteristics are useful in combination for distinguishing plethodontine salamanders from other plethodontids, although the characteristics listed are not synapomorphies of the subfamily Plethodontinae. Because Wake and Lombard’s (1986) analysis of 30 morphological characters predated the discovery of Karsenia (Min et al., 2005), the characteristics detailed below were scored for Karsenia only where noted.
Tongue and Hyobranchial Apparatus
Tongues are attached to the jaw by a short muscle plus connective and mucosal tissue. Tongues are protrusible (attached) in Aneides, (supergenus) Desmognathus, Karsenia, and Plethodon; projectile (attached) in Ensatina; and projectile (free) in Hydromantes. The hyobranchial skeleton includes a urohyal (except in Hydromantes), cylindrical basibranchial (except in Hydromantes which has an expanded basibranchial), and radii that are broad and flat and independent of the basibranchial (except in the supergenus Desmognathus, where the radii are rodlike structures of near constant diameter which retain their individuality, and in Hydromantes, where the radii have been lost). The basibranchial, first ceratobranchial and epibranchial elements are of approximately equal length, differing from Hydromantes, where the epibranchial is relatively longer, and the ceratobranchials are relatively shorter. The first ceratobranchial is the longest element in Karsenia, and the epibranchial is shorter than the basibranchial. The mostly ossified basibranchial of Karsenia has a pronounced anterior projection in front of the slender, elongate cornua. The first ceratobranchial is larger than the second ceratobranchial and constitutes the main force-transmitting element in movement of the tongue, with the exception of Hydromantes, where the the second ceratobranchial is larger in diameter than the first and constitutes the primary force-transmitting element. The rectus cervicis profundis muscle is linearly arranged, except in Hydromantes where the muscle is folded dorsally near its anterior end. The rectus cervicis superficialis has a lateral slip that has been lost in Hydromantes. The omohydoideus, genioglossus, circumglossus and basiradialis muscles are present in all taxa save for Hydromantes. Hydromantes is the only taxon in this clade that possesses a muscular projection cylinder. The intraglossus is attached to the anterior end of basibranchial, lingual cartilage, or equivalent, except in Ensatina, where it attaches instead to the anterior end of the glossal ligament, ventral to the basibranchial. The anterior section of the hyoglossus muscle has been lost, apart from the supergenus Desmognathus. The posterior fibers of the hyoglossus are oriented posteriorly, excepting Plethodon and Aneides where these fibers are oriented anteriorly. The suprapeduncularis muscle is well developed in Hydromantes, but weakly developed in other members of this clade. The ramus hypoglossus bifurcates distally, near the tip of the basibranchial, except in Ensatina where the bifurcation is posterior, between the attachment of the first ceratobranchial to the basibranchial and to the epibranchial; in Hydromantes, the supply to the tongue from the posterior bifurcationjoins with the ramus linguinalis branch of cranial nerve IX.

Skeleton of Aneides flavipunctatus (juvenile specimen) (red = bone; blue = cartilage). The hyobranchial skeleton is visible in blue at the anterior portion of the specimen. (Photograph © Allan Larson)
Epibranchial Number
Plethodon, Aneides, and Ensatina embryos have three epibranchials, members of the supergenus Desmognathus have four, and Hydromantes embryos have only one.
Tail Autotomy
In Plethodon, Aneides and Hydromantes, cutaneous wound healing occurs, there are 3 caudosacral vertebrae, the first caudal vertebra is normal (not specialized), and tail breakage is not localized. In the supergenus Desmognathus, cutaneous wound healing does not occur, there are 2 caudosacral vertebrae, the first caudal vertebra is normal and tail breakage is not localized. In Ensatina, cutaneous wound healing in the tail occurs, there are 3 caudosacral vertebrae, the first caudal vertebra is specialized, and tail breakage is localized.
Brain Stem Motor Column
There are two distinct classes of cells in the motor column of the neck and trunk, except in Hydromantes, which has only one class of cells.
Jaws, Cranial Osteology and Structure of the Inner Ear
In the supergenus Desmognathus, the jaws are unique in having a ligament extending from the jaw to the atlas vertebra. The vomer lacks the bony shelf anterior to the preorbital process, not including the supergenus Desmognathus. Parietal bones are relatively simple, scalelike structures except in the supergenus Desmognathus, where a deep, saddlelike groove is present that extends across the posterolateral portion of the bone to accommodate the ligament extending from the lower jaw to the atlas. In Plethodon, Aneides, and Ensatina, the facial lobe of the maxilla is located in a relatively posterior position, near the center of the pars dentalis; in the supergenus Desmognathus, the facial lobe extends into the area vacated by the prefrontal bones; in Hydromantes, the facial lobe lies in a position near the anterior end of the maxilla, with a distinct section of the pars dentalis extending anterior to it. The premaxillae surround an intermaxillary gland that lies directly behind the pars dentalis, except in the supergenus Desmognathus, where the premaxillary bones are fused and the intermaxillary gland is entirely surrounded and, occasionally, completely roofed by the expanded bony growth. In the inner ear, the periotic canal forms a ventral loop immediately after leaving the periotic cistern; Hydromantes differs in having a dorsal loop. The bore radius of the otic semiarticular ducts has a negative allometry with respect to body weight, although only slightly so in Ensatina; the growth of the ducts is nearly isometric in Hydromantes.
Chromosome Number
The diploid number of chromosomes is 28.
Development
Development is direct in Aneides, Ensatina, Hydromantes, Karsenia, Plethodon, and three species of the supergenus Desmognathus (Desmognathus aeneus, D. wrighti and Phaeognathus hubrichti). All remaining species of the supergenus Desmognathus belong to the genus Desmognathus and have biphasic life histories featuring gilled, aquatic larvae and fully metamorphosed adults.
Classification
Traditionally, Plethdontidae was subdivided into two subfamilies, Desmognathinae and Plethodontinae, the latter of which was further subdivided into the tribes Bolitoglossini, Hemidactyliini and Plethodontini (Wake, 1966; Lombard and Wake, 1986). Recent phylogenetic analyses using whole mitochondrial genomic DNA sequences (Macey, 2005; Mueller et al., 2004) and combined mitochondrial DNA, nuclear DNA and morphology (Chippindale et al., 2004) however, have found desmognathines to be nested within plethodontines.
As a result, Chippindale et al. (2004) recommended making the subfamily Plethodontinae equivalent to the former tribe Plethodontini plus Desmognathus and Phaeognathus, with those genera forming the supergenus Desmognathus within Plethodontinae. Similarly, Macey (2005) also recommended discontinuing recognition of the subfamily Desmognathinae, instead including that group within Plethodontinae along with Hydromantes, a taxon not included in the analysis of Chippindale et al. (2004).
Subsequently, Min et al. (2005) added the newly-discovered Karsenia koreana to the subfamily Plethodontinae based on morphological and molecular data.
Thus, the subfamily Plethodontinae (sensu Chippindale et al. [2004], Macey [2005], and Min et al. [2005]), comprises the genera Aneides, Ensatina, Hydromantes, Karsenia and Plethodon plus the supergenus Desmognathus. Although the closest relatives of Hydromantes remain to be determined, recent phylogenetic results place it within this clade (Mueller et al., 2004; Macey, 2005; Min et al., 2005).
Aneides contains six species noteworthy because of evolutionarily derived structures in the limbs and jaws (see introduction section above). Ensatina is currently recognized as one species, although some criteria would require that it be recognized as several different species (Frost and Hillis, 1990; Highton, 1990).
The genus Plethodon has been divided into eight species groups (Highton and Larson, 1979) based on statistical analysis of protein variation (Highton, 1991; 1995), which are supported by phylogenetic analysis of mitochondrial DNA sequence data (Mahoney, 2001).
Discussion of Phylogenetic Relationships
Phylogenetic relationships among plethodontines have been inferred from allozymic, immunological, DNA sequence, and morphological data (Highton and Larson, 1979; Maxson et al, 1979; Larson et al., 1981; Larson, 1984; Highton et al., 1989; Highton, 1991; Wynn et al., 1988; Chippindale et al., 2004; Mueller et al., 2004; Macey, 2005; Min et al., 2005). The most recent phylogenetic analyses have recovered relationships that contrast strongly with traditional taxonomic groupings based on morphology, e.g., the placement of desmognathines within plethodontines (Chippindale et al., 2004; Mueller et al., 2004; Macey, 2005; Min et al., 2005).
Determining the phylogenetic positions of Hydromantes, Ensatina, and Hemidactylium has been particularly problematic. Using partitioned Bayesian and maximum likelihood analyses of whole mitochondrial genomic DNA sequences, Mueller et al. (2004) found support for Ensatina as sister to Desmognathus + Phaeognathus. Hemidactylium was placed sister to Batrachoseps in partitioned Bayesian and maximum likelihood analyses, while maximum parsimony placed Hemidactylium either at the base of the tree or sister to all other plethodontids. Hydromantes was placed as sister to Aneides when employing a single model of nucleotide substitution, although this relationship was not supported using their preferred method of analysis employing different models of nucleotide substitution for different partitions of the data.
Similar to Mueller et al. (2004) Macey (2005) placed Hemidactylium as sister to all other plethodontids using maximum parsimony.
Using Bayesian and maximum parsimony analysis based on separate and combined analyses of morphological and molecular data, Chippindale et al. (2004) placed Ensatina sister to (Aneides, (Desmognathus,Phaeognathus)), with Hemidactylium sister to (Ensatina,(Aneides,(Desmognathus,Phaeognathus))). Hydromantes was not sampled in their study.
Min et al. (2005) investigated the phylogenetic position of Karsenia using Bayesian phylogenetic analysis of the nuclear-encoded RAG-1 gene. Their analysis placed Karsenia as the sister taxon to a clade comprising Aneides and members of the supergenus Desmognathus (sensu Chippindale et al., 2004). Ensatina was placed sister to Hydromantes, and together this clade was sister to (Karsenia(Aneides,Desmognathus)). Plethodon was sister to (Ensatina,Hydromantes(Karsenia(Aneides,Desmognathus))). Hemidactylium was placed in a polytomy with a clade comprising members of the Bolitoglossinae and another containing members of the Spelerpinae (sensu Chippindale et al., 2004).
References
Chippindale, P. T., R. M. Bonett, A. S. Baldwin, and J. J. Wiens. 2004. Phylogenetic evidence for a major reversal of life-history evolution in plethodontid salamanders. Evolution 58:2809-2822.
Dawkins, R. 2004. The Ancestor's Tale: A Pligrimage to the Dawn of Evolution. Houghton Mifflin Co., New York.
Dobzhansky, T. 1958. Species after Darwin. Pp. 19-55 in S. A. Barnett (ed.) A Century of Darwin. Harvard Univ. Press. Cambridge, Massachusetts.
Duellman, W. E. 1993. Amphibian Species of the World: Additions and Corrections. Univ. of Kansas Printing Service. Lawrence, KS.
Estes, R. 1981. Gymnophiona, Caudata. Handbuch der Paläoherpetologie 2:1-115.
Frost, D. R. 1985. Amphibian Species of the World. Allen Press and the Association of Systematics Collections. Lawrence, Kansas.
Frost, D. R. and D. M. Hillis. 1990. Species in concept and practice: Herpetological applications. Herpetologica 46:87-104.
Futuyma, D. J. 1998. Evolutionary Biology. Sinauer Associates, Inc., Sunderland, MA.
Garcia-Paris, M. and S. M. Deban. 1995. A novel anti-predator mechanism in salamanders: Rolling escape in Hydromantes platycephalus. Journal of Herpetology, 29, 149-151.
Gregory, T. R. 2006. Animal Genome Size Database. http://www.genomesize.com.
Highton, R. 1972. Distributional interactions among eastern North American salamanders of the genus Plethodon. Virginia Polytechnic Institute Research Division Monograph 4:139-188.
Highton, R. 1990. Taxonomic treatment of genetically differentiated populations. Herpetologica 46:114-121.
Highton, R. 1991. Molecular phylogeny of plethodonine salamanders and hylid frogs: Statistical analysis of protein comparisons. Molecular Biology and Evolution 8:796-818.
Highton, R. 1995. Speciation in eastern North American salamanders of the genus Plethodon. Annual Review of Ecology and Systematics 26:579-600.
Highton, R. and A. Larson. 1979. The genetic relationships of the salamanders of the genus Plethodon. Systematic Zoology 28: 579-599.
Highton, R., G. C. Maha and L. R. Maxson. 1989. Biochemical evolution in the slimy salamanders of the Plethodon glutinosus complex in the eastern United States. Illinois Biological Monographs 57:1-154.
Larson, A. 1984. Neontological inferences of evolutionary pattern and process in the salamander family Plethodontidae. Evolutionary Biology 17:119-217.
Larson, A., D. B. Wake, L. R. Maxson and R. Highton. 1981. A molecular phylogenetic perspective on the origins of morphological novelties in the salamanders of the tribe Plethodotini (Amphibia, Plethodontidae). Evolution 35:405-422.
Lombard, R. E. and D. B. Wake. 1986. Tongue evolution in the lungless salamanders, family Plethodontidae. IV. Phylogeny of plethodontid salamanders and the evolution of feeding dynamics. Systematic Zoology 35:532-551.
Mahoney, M. J. 2001. Molecular systematics of Plethodon and Aneides (Caudata: Plethodontidae: Plethodontini): Phylogenetic analysis of an old and rapid radiation. Molecular Phylogenetics and Evolution 18:174-188.
Maxson, L. R., R. Highton and D. B. Wake. 1979. Albumin evolution and its phylogenetic implications in the plethodontid salamander genera Plethodon and Ensatina. Copeia 1979:502-508.
Mead, L. S., D. R. Clayton, R. S. Nauman, D. H. Olson, and M. E. Pfrender. 2005. Newly discovered populations of salamanders from Siskiyou County California represent a species distinct from Plethodon stormi. Herpetologica 61:158-177.
Min, M. S., S. Y. Yang, R. M. Bonett, D. R. Vieites, R. A. Brandon, and D. B. Wake. 2005. Discovery of the first Asian plethodontid salamander. Nature 435:87-90.
Mueller, R. L., J. R. Macey, M. Jaekel, D. B. Wake, and J. L. Boore. 2004. Morphological homoplasy, life history evolution, and historical biogeography of plethodontid salamanders inferred from complete mitochondrial genomes. PNAS 101:13820-13825.
Reagan, N. L. 1992. Evolution of Sexual Isolation in Salamanders of the Genus Plethodon. Ph.D. dissertation. University of Chicago.
Sessions, S. K. and A. Larson. 1987. Developmental correlates of genome size in plethodontid salamanders and their implications for genome evolution. Evolution 41:1239-1251.
Staub, N. L. 1993. Intraspecific agonistic behavior of the salamander Aneides flavipunctatus (Amphibia: Plethodontidae) with comparisons to other plethodontid species. Herpetologica 49:271-282.
Stebbins, R. C.. 1949. Speciation in salamanders in the plethodontid genus Ensatina. University of California Publications in Zoology 48:377-526.
Stebbins, R. C. 1954. Natural history of the salamanders of the plethodontid genus Ensatina. University of California Publications in Zoology 54:47-124.
Wake, D. B. 1963. Comparative osteology of the plethodontid salamander genus Aneides. Journal of Morphology 113:77-118.
Wake, D. B. 1966. Comparative osteology and evolution of the lungless salamanders, family Plethodontidae. Memoirs of the Southern California Academy of Sciences 4:1-111.
Wake, D. B. 2006. Problems with species: patterns and processes of species formation in salamanders. Annals of the Missouri Botanical Garden 93:8-23.
Wake, D. B. and A. Larson. 1987. Multidimensional analysis of an evolving lineage. Science 238:42-48.
Wake, D. B., A. Salvador, and M. A. Alonso-Zarazaga. 2005. Taxonomy of the plethodondtid salamander genus Hydromantes (Caudata: Plethodontidae). Amphibia-Reptilia 26:543-548.
Weisrock, D. W., K. H. Kozak, and A. Larson. 2005. Phylogeographic analysis of mitochondrial gene flow and introgression in the salamander, Plethodon shermani. Molecular Ecology 14:1457-1472.
Wynn, A. H., R. Highton and J. F. Jacobs. 1988. A new species of rock-crevice dwelling Plethodon from Pigeon Mountain, Georgia. Herpetologica 44:135-143.
About This Page
David Heyse, Richard Highton and Todd Jackman contributed to the preparation of this Tree of Life page.
Allan Larson

Washington University, St. Louis, Missouri, USA
David Wake

University of California, Berkeley, California, USA
Tom Devitt

University of California, Berkeley, California, USA
Correspondence regarding this page should be directed to David Wake at
Page copyright © 2006 Allan Larson, David Wake, and
All Rights Reserved.
- Content changed 26 September 2006
Citing this page:
Larson, Allan, David Wake, and Tom Devitt. 2006. Plethodontinae. Version 26 September 2006 (under construction). http://tolweb.org/Plethodontinae/15533/2006.09.26 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site