Corydalus
Atilano Contreras-Ramos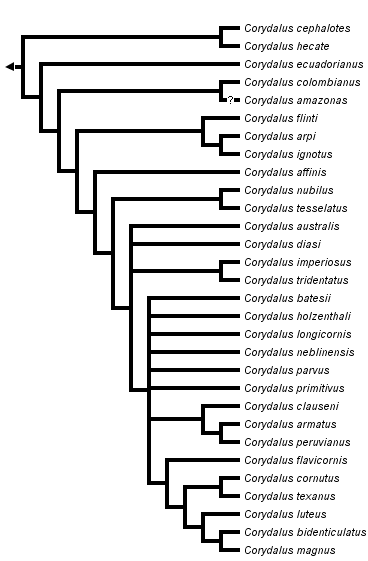

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The genus Corydalus Latreille is one of three currently recognized genera of New World dobsonflies (Megaloptera: Corydalidae: Corydalinae). Chloronia Banks and Platyneuromus Weele have been revised within the last 15 years (Penny and Flint 1982; Glorioso and Flint 1984; Flint 1991, 1992; Contreras-Ramos 1995). However Corydalus, the most species rich of all three was recently revised (Contreras-Ramos 1998), nearly 50 years after the last species was described in it.
Of the American dobsonflies, species of Corydalus are probably the best known. This is in part due to their large size (forewing length up to 85 mm), but also to the ubiquity of the larvae in many lotic environments and their widespread occurrence (from southeastern Canada to southeastern Brazil and northern Argentina). Most entomologists and lay people, however, remember this genus for the specialized elongate mandibles of the males of several species, a trait present (by convergence) in Asian Acanthacorydalis as well.
Thirty species of Corydalus are currently recognized, 22 of them exclusively of South American occurrence. A complete list of these species is available here. A few more species (at least five) remain undescribed, as they are known each from single female specimens.
Characteristics
Adult Corydalus are typically distinguished from Chloronia and Platyneuromus (the other two New World dobsonfly genera) by their general appearance. Heads of adult Platyneuromus have lateral postocular flanges (a platelike expansion of the head behind the eyes, more conspicuous in males), and individuals of all Chloronia species have a bright lemon yellow coloration of the wings. Adult Corydalus are typically reddish to grayish brown, some times pale brown or nearly black, often with small white spots on their forewings. Males of several species (all North and Central American species and some South American ones) show the characteristic modified mandibles (elongate with reduced dentition) which have an allometric relation with body size. Nevertheless, not all species have males with modified mandibles (some phylogenetically basal species are monomorphic), and so the 4 and 3-segmented maxillary and labial palps, respectively, remain as the only generalized diagnostic trait for the genus (although these traits are convergent in Chauliodinae).


A female dobson fly, Corydalus texanus Banks, Chihuahua, Mexico. Photograph copyright © 1997, Atilano Contreras-Ramos
Brief diagnosis. Forewing length 35-85 mm, generally 55-63 mm. Head typically unpatterned to slightly patterned, less frequently strongly patterned or with lateral vittae. Male mandibles often elongate with reduced dentition, some times unmodified. Maxillary and labial palps 4 and 3-segmented, respectively. Forewings generally pale to dark brown and moderately patterned with small white spots, seldom clear translucent or strongly patterned; last branch of Rs nearly always not bifurcate; M1+2 typically 2-branched (some times secondarily forked), rarely 4-branched. Male genitalia with 9th tergum nearly always with lateral bands of microsetae; 10th tergites typically elongate tubular, often apically incurved, seldom short or bent in abrupt angle; 9th gonostyli typically elongate subclavate, 10th sternite lobes generally papilliform. Female abdomen with sternal pouch between segments 6 and 7 well developed to reduced.
Taxonomic History
Linnaeus (1758) described the neuropteran Hemerobius cornutus, from Pennsylvania, calling attention to the strong dimorphism between male and female mandibles. Later this species was transferred to Corydalus by Latreille (1802), now in his simultaneously created order Megaloptera. Most early descriptions of Corydalus species (1839-1868) were by European workers, such as Burmeister, Rambur, Erichson, and MacLachlan, and the American entomologists Hagen and Banks. Davis (1903) and Weele (1910) performed revisionary works, however a few more species were described afterwards, notably by Stitz and Navás (1914-1936). Banks (1948) described the last species before the latest revision. Glorioso (1981) defined under a modern (cladistic) protocol the genera of World dobsonflies, including Corydalus. Penny (1993) and Contreras-Ramos (1998) have further reevaluated Corydalus' limits in the context of phylogenetic analyses.
Natural History
Most of what is known about Corydalus life history is from the Nearctic (e.g., Parfin 1952, Evans 1972, Stewart et al. 1973, Brigham 1982, Bowles 1990, Evans and Neunzig 1996), with little comparable information for Neotropical species (e.g., Geijskes 1984; Roldán Pérez 1988; Contreras-Ramos 1997, 1999; Henry et al. 1992). Several studies have been contributed for Japanese species of Protohermes (e.g., Hayashi 1994), which could be models to follow for other faunas.
Adults of Corydalus are nocturnal and secretive, seldom seen during day time as they hide under leaves in the canopy. With some luck they are found in aggregates under bridges or other structures near lotic environments (the general habitat for all dobsonflies). However Corydalus adults, more often females, are attracted to lights, and light traps (black light, mercury vapor) are a typical means for their capture. In northern Mexico, it is not uncommon to find individuals of C. luteus (Hagen) (see title picture) under street lights of small towns or resting between bricks on houses near a stream. At the right time of the year (May-June) females can be found at night on rocky walls of streams, ovipositing, and during the day one can see the chalky white egg masses (coin size) as evidence of these activities. Some times there are hundreds of spots of former egg masses as proof of many years of dobsonfly occurrence. Obviously a female dobsonfly is capable of selecting such ovipositing sites, since they would abandon formerly used walls as streams change their course (e.g., after a hurricane). Adults most likely will not feed, but they are known to drink sweet solution in captivity. Life span is about a week in captivity, but it might be longer in natural conditions. Mating behavior appears to be stereotyped (males performing wing fluttering and displaying genitalic appendages in an "arrogant" posture, as well as fighting over females). It can be observed in captivity, preferentially in a large cage conditioned to imitate the natural habitat, and using a red light.
Upon hatching, first instar larvae fall into the water or seek the aquatic environment. There, larvae live for 1-5 years (in the Nearctic), undergoing 10-12 instars in a generally univoltine cycle (seldom semivoltine). In the Neotropics larval development probably takes about a year, but in colder (higher elevation) streams it might take longer (there are no studies demonstrating this, and there might be a possibility also for a bivoltine cycle in constantly warm climate streams). Larvae are generalist predators and appear to be more active at night. It is interesting that dobsonfly larvae obtain dissolved oxygen through abdominal lateral filaments and tracheal gills, but have also spiracles that allow them for direct intake of air. This should be a survival advantage for larvae inhabiting intermittent streams in arid regions. For instance, I have found larval aggregates of C. luteus under rocks, sharing the humid stream bed with crayfishes in northeastern Mexico, helplessly awaiting for rains. A similar condition might occur with C. affinis (Burmeister) in the Chaco of northern Argentina (Dr. A. O. Bachmann, Museo Argentino de Ciencias Naturales, in litt.)


Male pupa of Corydalus cornutus (L.), Alabama, USA. Photograph copyright © 1997, Atilano Contreras-Ramos
When mature, larvae (prepupae) leave the water and select a rock or log where they build a chamber for pupation. This happens typically close to the water, but I have found prepupae of a related genus (Platyneuromus), as far as 20 m uphill into the forest away from the stream. This would make pupae nearly impossible to be found in a dense tropical forest. After a few days as prepupae and several more as pupae (probably about a week), the adults emerge from the pupating chamber (where they leave their larval and pupal skins) and the cycle is completed. Much remains to be investigated regarding adult behavior (e.g., mating behavior and chemical communication between sexes), larval ecology (e.g., habitat distribution and potential segregation in sympatry), functional morphology, seasonality and patterns of emergence, description of immature stages, etc.
Discussion of Phylogenetic Relationships
Monophyly of Corydalus
The phylogeny presented above is a strict consensus tree from three equally parsimonious trees (n char = 120, tree length = 439, consistency index = 0.405, retention index = 0.634, rescaled consistecy index = 0.257) included in the genus revision (Contreras-Ramos 1998a). The monophyly of all species grouped within Corydalus is well supported by two characters (convergent in Chauliodinae), a reduction in the number of maxillary and labial palp segments, from 5 to 4 and from 4 to 3 respectively (Glorioso 1981, Penny 1993, Contreras-Ramos op. cit.). Other characters that provide support to such grouping are somewhat homoplasious, for instance male 9th gonostyli and 10th tergites subequal in length, labral position between mandibles, vein Rs as a single vein, and non unguiform 9th gonostyli. Characters that might be synapomorphies for Corydalus are the unequal relative size of mandibular preapical teeth, clypeus clearly fused to frons, anterior margin of submentum dorsoventrally developed, and moderately to well developed submental projections (Contreras-Ramos op. cit.).
General Trends
A major division occurs between a phenotypically cohesive monophyletic group ("typical" Corydalus species; C. australis, C. diasi, and all other species up to C. magnus) and a rather heterogeneous paraphyletic grouping, the "atypical" Corydalus species (C. cephalotes, C. hecate, up to C. nubilus and C. tesselatus). An explanation for this pattern might be that the upper group represents younger lineages of closely related species (e.g., species within the C. armatus and the C. cornutus species groups), whereas the lower species are either remnants of former diverse clades or have evolved in isolation for a considerably long time (and so developing their distinct morphologies). It is, however, conceivable that additional species related to some of the "atypical" basal species or groups might be discovered, thus converting distinct species morphologies (e.g., that of C. tesselatus) into distinct species group morphologies.
Selected Species Groups
Several species pairs or groups were supported in all three equally parsimonious trees. For instance, the C. cephalotes species pair, the C. arpi species group, the C. nubilus species pair, the C. tridentatus species pair, the C. armatus species group, and the C. cornutus species group. Overall these groups or sister group relationships are well supported. As examples, the C. arpi species group is supported by (among other characters) strongly convex lateral corners of frons, antennae distinctly colored in three sections, and reduced 10th sternite lobes; the C. armatus species group is supported by two unambiguous character changes, well developed anterolateral apodemes of the 10th sternite and 10th sternite lobes directed posteroventrally; and the C. cornutus species group (which includes C. flavicornis up to C. magnus and C. bidenticulatus) is supported by strongly separated basal preapical teeth (in female mandibles) and a reversal to slightly elongate papilliform 10th sternite lobes (also present in C. ecuadorianus).
Phylogenetic Position of Corydalus amazonas
This species, described based only on females, was excluded from the original phylogenetic analysis due to the many characters missing. When included, five equally parsimonious trees were obtained (tree length = 447, consistency index = 0.398, retention index = 0.630, rescaled consistecy index = 0.251). In all five trees C. amazonas emerged as sister to C. colombianus, either in the position shown above or basal to C. affinis (i.e., as sister to C. affinis and rest of the species). Corydalus amazonas overall aspect (e.g., robust head, wing shape, bifurcate last branch of Rs) is similar to C. cephalotes and C. hecate. At least, it seems quite likely C. amazonas falls within the atypical species (below the node of C. australis, C. diasi, etc.)
Biogeographical and Evolutionary Considerations
Descriptive Biogeography
Corydalus is the most widespread genus of American dobsonflies. It occurs from southeastern Canada south through most of North America, Central America, and a large portion of South America. It is absent from the Greater and Lesser Antilles, and roughly from most of southern South America including the Chilean, Subantarctic, Patagonian, and southern half of Pampean Provinces (sensu Cabrera & Willink 1980). The majority of Corydalus species are South American, and can roughly be subdivided into those with non Andean eastern distributions (C. cephalotes, C. hecate, C. amazonas, C. flinti, C. arpi, C. ignotus, C. affinis, C. nubilus, C. batesii, C. neblinensis, C. imperiosus, C. tridentatus, C. diasi, and C. australis), and those associated with the Andes (C. primitivus, C. sp. holzenthali, C. parvus, C. longicornis, C. armatus, C. ecuadorianus, C. colombianus, and C. tesselatus). A few species are mostly South American (Andean) with extensions into Central America (C. peruvianus, C. flavicornis, and C. clauseni). Finally, a group of species occurs in Central and North America (C. luteus, C. magnus, C. bidenticulatus, C. texanus, and C. cornutus).
Historical Biogeography
The pattern of distribution of dobsonfly genera (absence of dobsonflies in Europe and neighboring Asia, and nearly all of Africa), the distant relatedness of the New World lineage and Chloroniella (from South Africa), and the presence of a modern lineage in North America, suggest that an early isolation of the New World lineage might have taken place (perhaps as early as Pangean times or early Triassic). The most basal Corydalus species (C. cephalotes and C. hecate) are endemic to southeastern Brazil (as are the two apparently most basal Chloronia species), however the next two species in the phylogeny (C. ecuadorianus and C. colombianus) have a distant northern Andean distribution. Such pattern indicates an old southeast-northwest vicariance event, probably associated with the uplifting of the Andes during upper Mesozoic. Another early isolation event (perhaps related to the development of the Guiana Highlands) was probably responsible for the origin and differentiation of the C. arpi species group. Other species may have originated as a result of the continuous orogenic activity that took place in northwestern South America throughout the Cenozoic. Phylogeny suggests the dispersal of a species (ancestral to the C. cornutus species group) from South America into Central and North America, with further differentiation into two groups (the sister C. cornutus and C. texanus, North American; and the group formed by C. luteus, C. magnus, and C. bidenticulatus, mostly Mexican and Central American).
Diversification in Corydalus
As stated by Eberhard (1985), in most insect groups genitalia have evolved rapidly and divergently and are used as taxonomic characters to distinguish species. However, in some insects, male genitalia typically do not show species-specific variations. In the first case, intrommitent organs, internal transfer of sperm, and associated complexity in morphology would be part of a process of sexual selection by female choice. Dobsonflies, including Corydalus, seem to fall in the second category. This is supported by the absence of intromittent organs (i.e., a large, gelatinous spermatophore is used instead; Hayashi 1992) and a trend for similar male genitalic morphology in related species (e.g., by insect standards male genitalia of C. primitivus and C. luteus are remarkably similar). This reproductive mode, in addition to a generalized life history (e.g., generalist predaceous larvae with euryoecious habits), might have resulted in a low number of morphologically similar species, often also widely distributed.
References
Baker, J. R., and H. H. Neunzig. 1968. The egg masses, eggs, and first-instar larvae of eastern North America Corydalidae. Ann. Ent. Soc. Amer. 61: 1181-1187.
Bowles, D. E. 1990. Life history and variability of secondary production estimates for Corydalus cornutus (Megaloptera: Corydalidae) in an Ozark stream. J. Agric. Entomol. 7: 61-70.
Brigham, W. U. 1982. Megaloptera, pp. 7.1-7.12 In A. R. Brigham, W. U. Brigham, and A. Gnilka (eds.). Aquatic insects and oligochaetes of North and South Carolina. Midwest Aquatic Enterprises, Mahomet, Illinois. [837 pp.]
Cabrera, A. L., and A. Willink. 1980. Biogeografía de América Latina. Organización de los Estados Americanos, Monografías Científicas, Ser. Biología 13: 1-122.
Chandler, H. P. 1956. Megaloptera, pp. 229-233 In R. L. Usinger (ed.). Aquatic insects of California. University of California Press, Berkeley. 508 pp.
Contreras-Ramos, A. 1997. Megaloptera, pp. 355-359 In E. González S., R. Dirzo, and R. C. Vogt (eds.). Historia natural de Los Tuxtlas. Universidad Nacional Autónoma de México, Mexico City. 647 pp.
Contreras-Ramos, A. 1998. Systematics of the dobsonfly genus Corydalus Latreille (Megaloptera: Corydalidae). Thomas Say Monographs, Entomological Society of America. Lanham, MD. 360 pp.
Contreras-Ramos, A. 1999. Mating behavior of Platyneuromus (Megaloptera: Corydalidae), with life history notes on dobsonflies from Mexico and Costa Rica. Ent. News 110: 125-135.
Contreras-Ramos, A., and S. C. Harris. 1998. The immature stages of Platyneuromus (Corydalidae), with a key to the genera of larval Megaloptera of Mexico. J. N. Am. Benthol. Soc. 17(4): 489-517.
Cuyler, R. D. 1956. Taxonomy and ecology of larvae of sialoid Megaloptera of east-central North Carolina with key to and descriptions of larvae of genera known to occur in the United States. M. S. Thesis. North Carolina State College, Raleigh. 150 pp.
Davis, K. C. 1903. Sialididae of North and South America. Bull. N. Y. State Mus. 68: 442-486, 499, 2 pls.
Eberhard, W. G. 1985. Sexual selection and animal genitalia. Harvard University Press, Cambridge. 244 pp.
Epler, J. H., and C. L. de la Rosa. 1995. Tempisquitoneura, a new genus of neotropical Orthocladiinae (Diptera: Chironomidae) symphoretic on Corydalus (Megaloptera: Corydalidae). J. N. Am. Benthol. Soc. 14(1): 50-60.
Evans, E. D. 1972. A study of the Megaloptera of the Pacific coastal region of the United States. Unpublished Ph. D. dissertation. Oregon State University, Corvallis. 210 pp.
Evans, E. D., and H. H. Neunzig. 1996. Megaloptera and aquatic Neuroptera, pp. 298-308 In R. W. Merritt and K. W. Cummins (eds.). Aquatic insects of North America. Kendall/Hunt Publishing Company, Dubuque, Iowa. 862 pp.
Flint, O. S., Jr. 1977. Neuroptera, pp. 187-188 In S. H. Hurlbert (ed.). Biota acuática de Sudamérica austral. San Diego State University, San Diego. 342 pp.
Geijskes, D. C. 1984. Notes on Megaloptera from the Guyanas, S. Am., pp. 79-84 In J. Gepp, H. Aspöck, and H. Hözel (eds.). Progress in World's Neuropterology, Grazz. 265 pp.
Glorioso, M. J. 1981. Systematics of the dobsonfly subfamily Corydalinae (Megaloptera: Corydalidae). Syst. Entomol. 6: 253-290.
Gurney, A. B., and S. I. Parfin. 1959. Neuroptera, pp. 973-980 Inn W. T. Edmondson (ed.). Fresh-water Biology. John Wiley & Sons, New York. 1248 pp.
Haldeman, S. S. 1848. History and transformations of Corydalus cornutus. Memoirs of the American Academy of Arts and Sciences 4: 157-161.
Hayashi, F. 1992. Large spermatophore production and consumption in dobsonflies Protohermes (Megaloptera, Corydalidae). Jpn. J. Ent. 60: 59-66.
Henry, C. S., N. D. Penny, and P. A. Adams. 1992. The neuropteroid orders of Central America (Neuroptera and Megaloptera), pp. 432-458 In D. Quintero and A. Aiello (eds.). Insects of Panama and Mesoamerica. Oxford University Press, Oxford. 692 pp.
Herrmann, S. J., and H. L. Davis. 1991. Distribution records of Corydalus cornutus (Megaloptera: Corydalidae) in Colorado. Ent. News 102: 25-30.
Mangan, B. P. 1994. Pupation ecology of the dobsonfly Corydalus cornutus (Corydalidae: Megaloptera) along a large river. J. Freshwat. Ecol. 9(1): 57-62.
Neunzig, H. H., and J. R. Baker. 1991. Order Megaloptera, pp. 112-122 In F. W. Stehr (ed.). Immature insects, Vol. 2. Kendall/Hunt Publishing Co., Dubuque, Iowa. 975 pp.
New, T. R., and G. Theischinger. 1993. Megaloptera (Alderflies, Dobsonflies). Handbuch der Zoologie, Vol. 4 (Part 33). Walter de Gruyter, Berlin. 97 pp.
Parfin, S. I. 1952. The Megaloptera and Neuroptera of Minnesota. The American Midland Naturalist 47: 421-434.
Penny, N. D. 1977. Lista de Megaloptera, Neuroptera e Raphidioptera do México, América Central, ilhas Caraíbas e América do Sul. Acta Amazonica 7(4): Suplemento, 61 pp.
Penny, N. D. 1981. Neuroptera, pp. 89-91 In S. H. Hurlbert, G. Rodríguez, and N. Dias dos Santos (eds.). Aquatic biota of tropical South America, Part 1. Arthropoda. San Diego State University, San Diego. 323 pp.
Penny, N. D. 1982. Neuroptera of the Amazon Basin. Part 7, Corydalidae. Acta Amazonica 12: 825-837.
Penny, N. D. 1982. Neuroptera, pp. 280-282 In S. H. Hurlbert and A. Villalobos-Figueroa (eds.). Aquatic biota of Mexico, Central America and the West Indies. San Diego State University, San Diego. 529 pp.
Riley, C. V. 1873. The hellgrammite fly Corydalus cornutus (Linn.), pp. 142-145 In Fourth, Fifth and Sixth annual reports on the noxious, beneficial and other insects of the state of Missouri. Reprint edition (1978). Arno Press, N. Y.
Rosa, C. L., de la. 1992. Phoretic associations of Chironomidae (Diptera) on Corydalidae (Megaloptera) in northwestern Costa Rican streams. J. N. Am. Benthol. Soc. 11: 316-323.
Short, R. A., E. H. Stanley, J. W. Harrison, and C. R. Epperson. 1987. Production of Corydalus cornutus (Megaloptera) in four streams differing in size, flow, and temperature. J. N. Am. Benthol. Soc. 6: 105-114.
Stewart, K. W., G. P. Friday, and R. E. Rhame. 1973. Food habits of hellgrammite larvae, Corydalus cornutus (Megaloptera: Corydalidae), in the Brazos River, Texas. Ann. Ent. Soc. Am. 66: 959-963.
Weele, H. W., van der. 1910. Megaloptera (Latreille), monographic revision In Collections Zoologiques du Baron Edm. de Selys Longchamps Fasc. V (Première partie), Bruxelles. 93 pp.
Title Illustrations

| Scientific Name | Corydalus luteus |
|---|---|
| Location | Nuevo León, Mexico |
| Sex | Male |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1997

|
About This Page
Acknowledgements. I wish to thank all the people that gave me help and encouragement through my graduate school work on Megaloptera, including: Nancy Adams, Roger Blahnik, Dave Bowles, Joaquín Bueno, Phil Clausen, Ollie Flint, Steve Harris, Fumio Hayashi, Ralph Holzenthal, Bill Miller, Margot Monson, Fernando Muñoz, Jola Nunley, Norman Penny, and Ray Pupedis. Many thanks to Roger Blahnik and John Oswald for editorial suggestions to three pages (Megaloptera, Corydalinae, and Corydalus) and the Megaloptera page, respectively. Thanks also to Dave Maddison and Katja Schulz, for their encouragement and supervision during construction of ToL pages.

Universidad National Autónoma de México
Correspondence regarding this page should be directed to Atilano Contreras-Ramos at
Page copyright © 1997
 Page: Tree of Life
Corydalus.
Authored by
Atilano Contreras-Ramos.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Corydalus.
Authored by
Atilano Contreras-Ramos.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 12 November 1997
- Content changed 15 November 1997
Citing this page:
Contreras-Ramos, Atilano. 1997. Corydalus. Version 15 November 1997. http://tolweb.org/Corydalus/12995/1997.11.15 in The Tree of Life Web Project, http://tolweb.org/










 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site